Biopharmaceutical companies, being in a regulated industry, have always been focused on meeting quality requirements and controlling their supply chain. However, in the past few years they have had to deal with increasingly stringent regulatory demands to demonstrate that they are in control of not only their internal manufacturing processes, but the manufacturing processes of their raw material vendors as well. In April 2013, we asked our panel of upstream and downstream bioprocessing contacts what they currently valued most in the quality systems of their raw material vendors. The results (see figure below) reflect the importance of raw material traceability, manufacturing process transparency and technical support to biopharmaceutical manufacturers and those that regulate them. The results also seem to indicate that avoiding problems in the first place is considerably more important than having systems in place to deal with them when they occur.
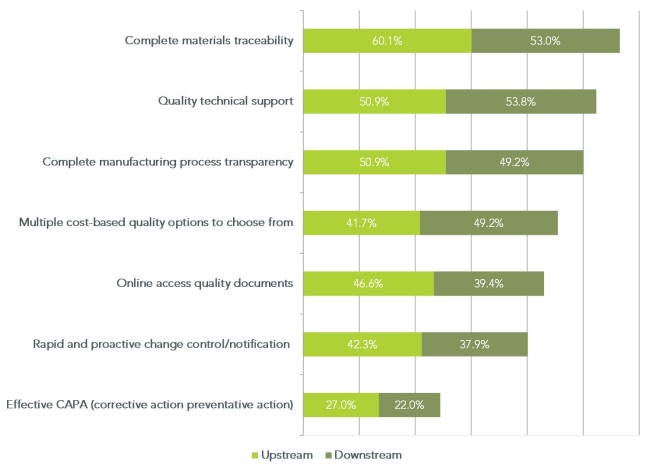
Q. What are your top three criteria for an “ideal quality systems program” for suppliers of bioproduction raw materials and services?
This suggests that vendors that are the original raw material manufacturers may be more attractive to biopharmaceutical companies relative to those that mostly OEM the raw material. Unlike the companies that don’t manufacture the raw materials, original manufacturers have direct control over the traceability and manufacturing processes for the raw materials they distribute. Provided they are willing to share this information with their customers, this cuts out a lot of extra supply chain management complexity and garners greater confidence in the raw material supplier.
Does this mean vertical integration within bioproduction raw material vendors is in vogue? Maybe. For biopharmaceutical companies the choice to outsource or internalize a process is and should be based on the circumstances at hand. Why should the raw materials suppliers act any differently?
Have unanswered questions about what biopharmaceutical companies in North America, Europe and Asia value in a vendor? We can help get the answers you need. Contact us here.


Leave a Reply